how to draw molecular orbital diagram for heteronuclear molecules
Heteronuclear Diatomic Molecules are composed of 2 different elements bonded together. F will be lower on the diagram.
Now more difficult MO diagrams can be derived using the four simple step above.
. The orbitals of different energies overlap in heteronuclear diatomic molecules. Molecular Orbital Diagrams of Heteronuclear Diatomics Answers 173 KB. LCAO of Heteronuclear Diatomics Answers 110 KB.
Molecular Orbital Diagrams of Heteronuclear Diatomics. FUNDAMENTAL STEPS IN DERIVING MO DIAGRAMS Find the valence electron configuration of each atom in the molecule. Fill the MOs with electrons.
The 2p-like character of the 3s molecular orbital in both CH and HF is evident in the density diagrams. Molecular Orbital Diagrams of Heteronuclear Diatomics. Draw tiny updown arrows for each electron that the atom possesses overall.
FUNDAMENTAL STEPS IN DERIVING MO DIAGRAMS. Orbital interactions to produce bonding or antibonding orbitals in heteronuclear diatomics occur if there is sufficient overlap between atomic orbitals as determined by their. Diagrams like these change according to if youre working with homonuclear molecules - molecules made of one element - or heteronuclear molecules - molecules made of different elements.
In terms of the forces exerted on the nuclei the 2 s charge density is strongly binding for both C and H while the 3 s charge density is only very weakly binding for H and is actually antibinding for. Shields shows you how to draw the MO correlation diagram for cyanide CN- calculate the MO bond order and write the MO electron configuration with an. Keep the energies of atomicmolecular orbitals when arranging the diagram.
Find the valence electron configuration of each atom in the molecule. How to draw molecular orbital diagram for heteronuclear molecules Friday February 25 2022 Edit Chem 101 Creating A Molecular Orbital Diagram For A Diatomic Ion In The Second Row With Aleks Youtube. You should know this from General Chemistry all three 2ps are degenerate.
In CH the 1 s orbital of H interacts strongly with both the 2 s and 2 p s orbitals on C. LCAO of Heteronuclear Diatomics. 2sa 2pa C 2sb 2pb CO O σ σ π π σ σ Bonding orbitals get polarized towards oxygen Anti-bonding orbitals get polarized towards carbon HOMO is on carbon LUMO is on carbon too.
While MOs for homonuclear diatomic molecules contain equal contributions from each interacting atomic orbital MOs for heteronuclear diatomics contain different atomic orbital contributions. Do the number of AOs number of MOs. Fill the MOs with electrons.
Fill molecular orbitals using energy and bonding properties of the overlapping atomic orbitals. The correct order of the orbitals depends on the s-p energy gap in both atoms the relative energetic distance of the s and p orbitals on both atoms respectively the orbital overlapp etc. You may try this yourself using eg.
ExampleN OCOHF N O C O H F forms the heteronuclear diatomic. The further to the right your element is the lower its energy levels are. Fill molecular orbitals using energy and bonding properties of the overlapping atomic orbitals.
Molecular Orbital Diagrams of Heteronuclear Diatomics Concept 1. So to obtain the correct ordering will require a calculation. 12-12 This video describes the molecular orbital theory diagram of CO placing emphasis on how MO theory differs for homo and heteronuclear diatomics.
See full answer below. Use the diagram to predict properties of the molecule. Some Extended Hueckel Calculators.
Thus the rule becomes. F will be lower on the diagram. Now MO diagrams are only simple for elements of the second row of the periodic table ceLi through ceNe.
Involving heavier atoms makes it harder to guess at molecular orbital diagrams and there is need for quantum chemistry calculations. Decide if the molecule is homonuclear of heteronuclear. These however also have their own depth.
Draw a vertical arrow labeled E for energy on the left side of a piece of paper oriented portrait rather than landscape and draw one horizontal line for each orbital on the energy scale. Do the number of AOs number of MOs. CO In molecules with more than one type of atom MOs are formed from AOs that have different energies.
Decide if the molecule is homonuclear of heteronuclear. To make sense of the complexities introduced by antibonding we build molecular orbital diagrams. Use the diagram to predict properties of the molecule.
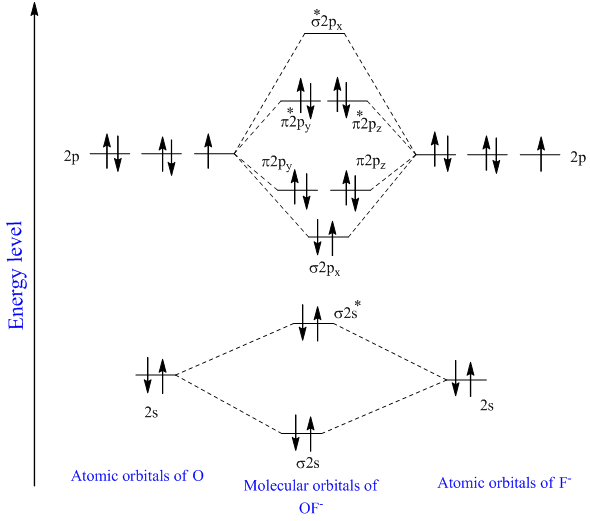
Solved Chapter 5 Problem 10p Solution Inorganic Chemistry 5th Edition Chegg Com

8 Drawing Molecular Orbital Diagrams Flux Science
Molecular Orbital Theory Diatomic Molecules Heteronuclear Molecules Chem
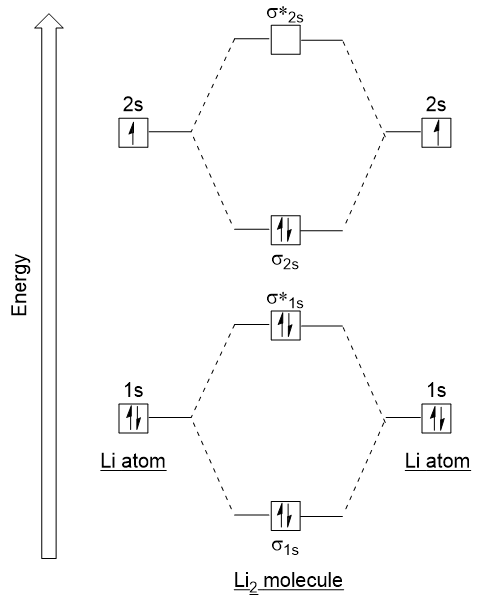
Molecular Orbitals Introductory Chemistry 1st Canadian Edition
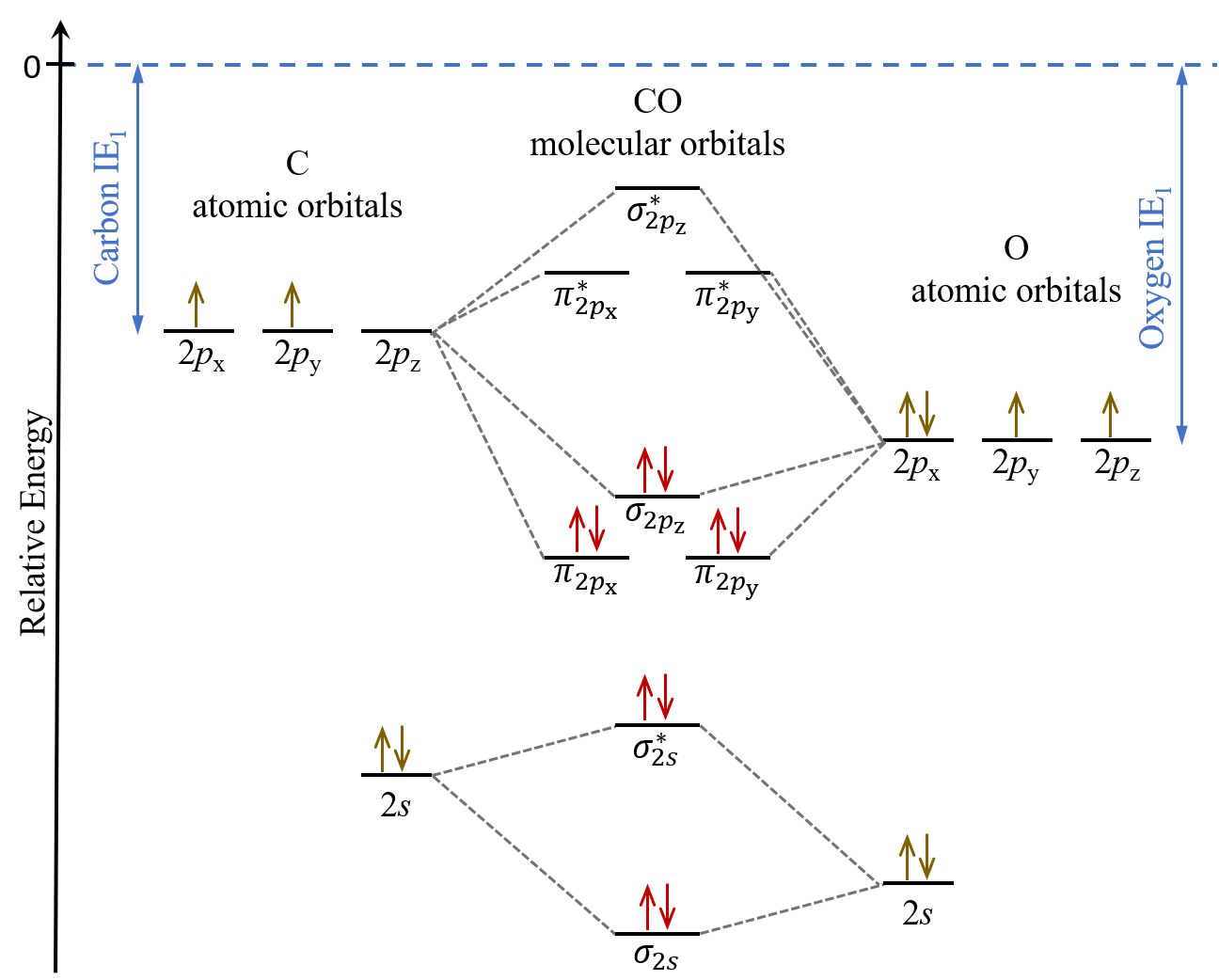
D6 5 Mos For Heteronuclear Diatomic Molecules Chemistry 109 Fall 2021

Molecular Orbital Diagrams Simplified By Megan A Lim Medium
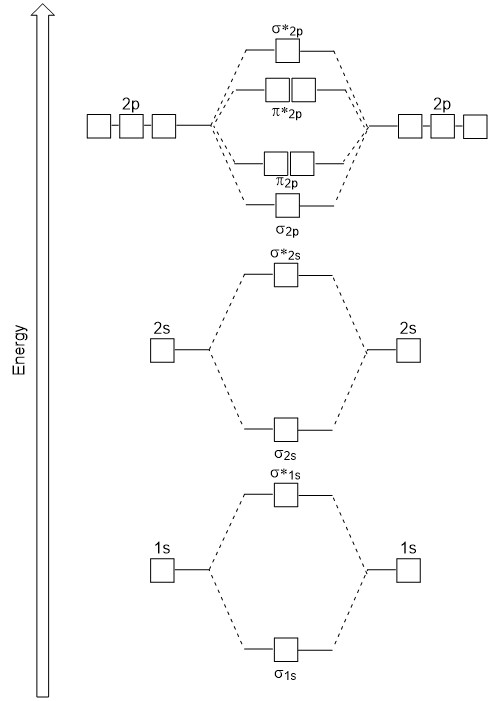
8 Drawing Molecular Orbital Diagrams Flux Science

8 5 Molecular Orbital Theory Chemistry
Molecular Orbital Diagrams Chemistry X Youtube
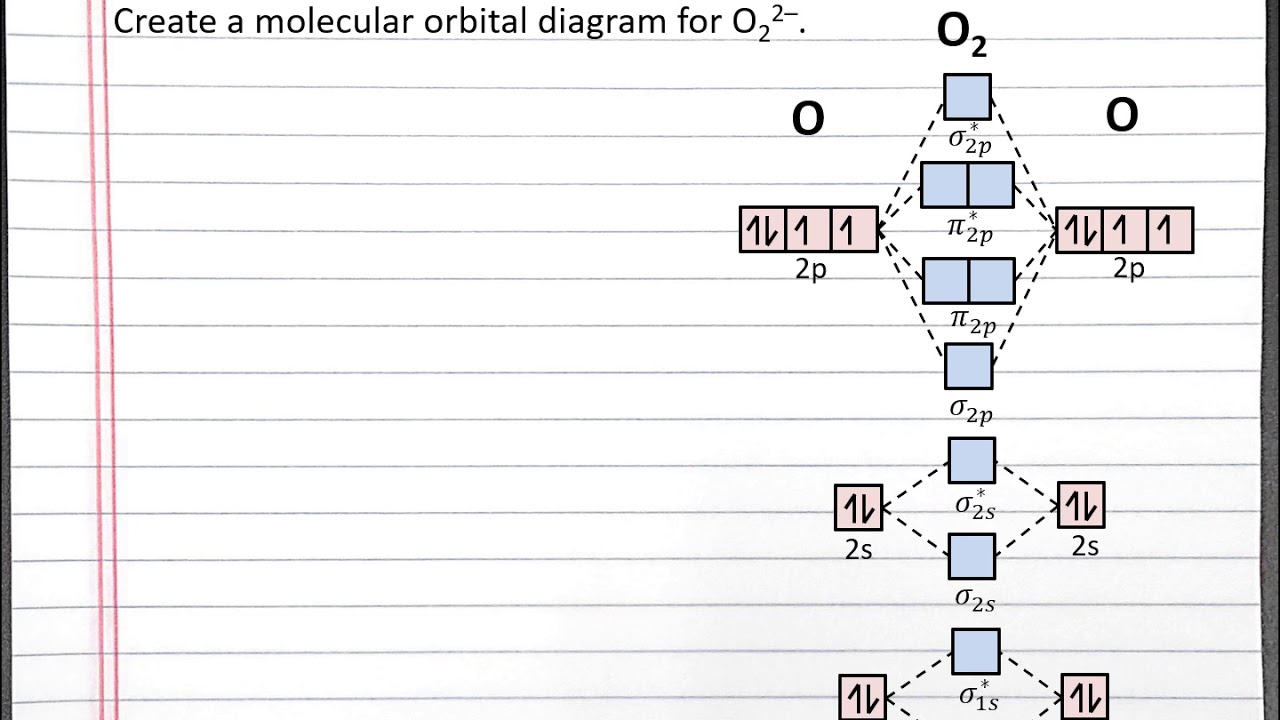
Chem 101 Creating A Molecular Orbital Diagram For A Diatomic Ion In The Second Row With Aleks Youtube

Molecular Orbital Diagram Of Polyatomic Co2 Molecules Chemical Bonding Molecular Structures Youtube

Solved Draw A Molecular Orbital Diagram For Each Of The Chegg Com

2 6 Molecular Orbital Theory Chemistry Libretexts
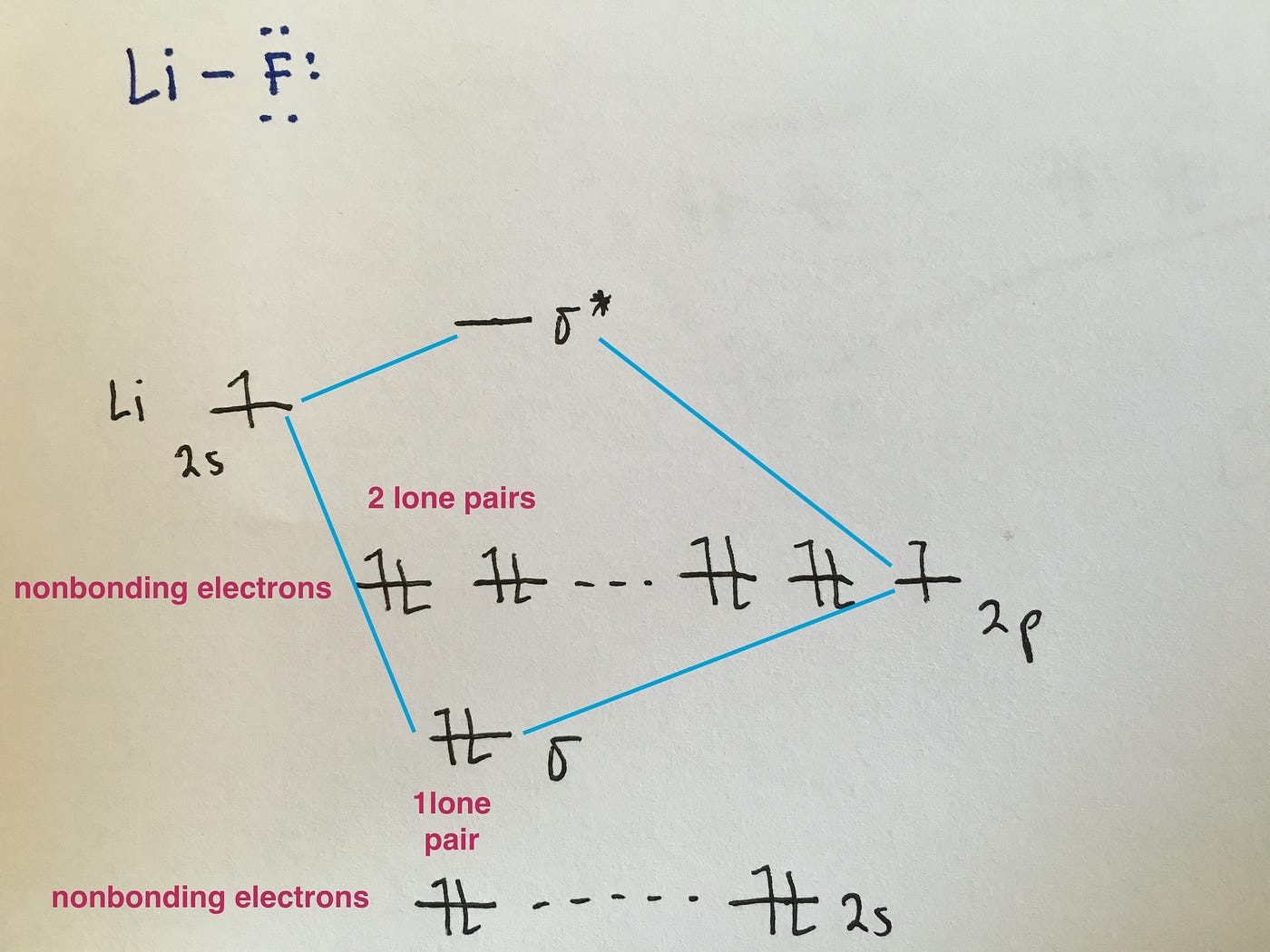
Molecular Orbital Diagrams Simplified By Megan A Lim Medium

Molecular Orbital Theory Heteronuclear Diatomic Cyanide Cn Example Youtube
Molecular Orbitals Introductory Chemistry
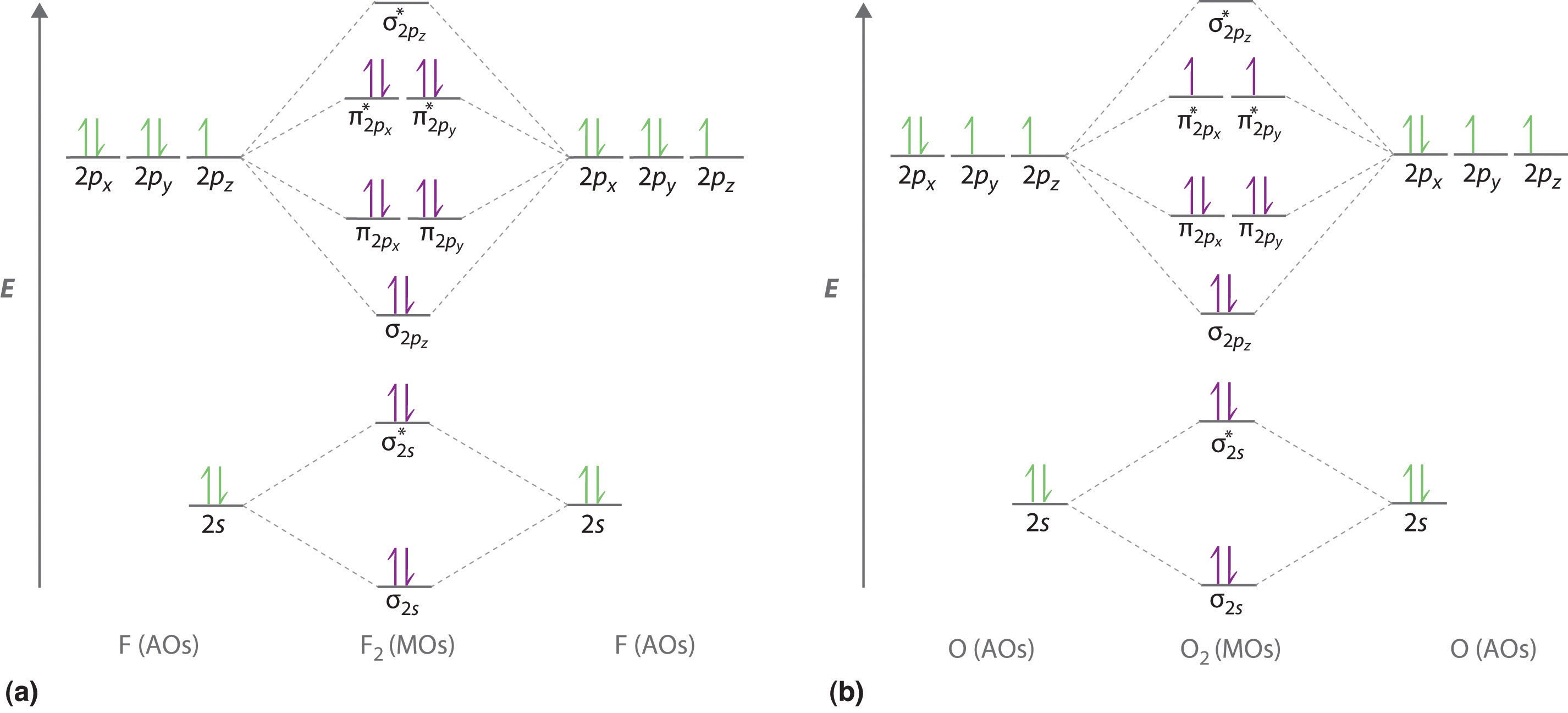
2 6 Molecular Orbital Theory Chemistry Libretexts

Question 13 Molecular Orbitals For Heteronuclear Diatomic Molecules Using The Above Molecular Orbital Diagram For Co Homeworklib